Edited by: Elissa Cameron
Online ISSN:1469-7998
Print ISSN:0952-8369
Volume 326
Issue 4
August 2025
THERAPEUTIC EFFECTS OF HONEY AND BEE VENOM ON BLOOD PARAMETERS, LIVER AND KIDNEY FUNCTION MARKERS, AND HISTOPATHOLOGICAL CHANGES IN METHOTREXATE-INDUCED TOXICITY IN ALBINO RATS
Authors :
Bhalchandra Baburao Waykar
DEPARTMENT OF ZOOLOGY, BABASAHEB AMBEDKAR MARATHWADA UNIVERSITY, CHHATRAPATI SAMBHAJINAGAR-431004, MAHARASHTRA, INDIA
Corresponding Author
Dhanya Shrijith Pillai
DEPARTMENT OF ZOOLOGY, BABASAHEB AMBEDKAR MARATHWADA UNIVERSITY, CHHATRAPATI SAMBHAJINAGAR-431004, MAHARASHTRA, INDIA
Abstract
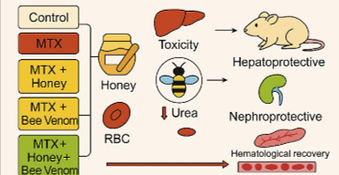
A popular chemotherapeutic and immunosuppressive drug, methotrexate (MTX), has restricted clinical utility due to its hepatotoxicity, nephrotoxicity, hematological suppression. Natural bioactive substances, like honey and bee venom, may have therapeutic promise as molecules that counteract MTX-induced toxicity since they are known to have immunomodulatory, anti-inflammatory, and antioxidant properties that can be therapeutic for reducing MTX-induced toxicity. This study aimed to see if honey and bee venom could shield albino rats from the toxicity of MTX. Animals were randomly assigned into five groups: normal control, MTX-treated, honey plus methotrexate-treated, bee-venom plus methotrexate treated, and combination-treated (honey plus bee venom plus methotrexate). Various parameters like body weight, liver function markers (Total protein, SGPT, SGOT, ALP, albumin, bilirubin), kidney function markers (creatinine, urea, uric acid), hematological indices (WBCs, RBCs, platelets, % hemoglobin (Hb), and mean values MCV, MCH and MCHC), and histopathology of liver and kidney were performed. Administration of MTX significantly increased liver and kidney function markers, as well as decreased hematological indices. The liver albumin was significantly decreased in the MTX group and treatment with honey and bee venom played a protective role. Honey and bee venom treatment resulted in significant improvements in all parameters and the combined effect of honey and bee venom with the drug marked most protective effects. It was shown that honey and bee venom possess hepatoprotective, nephroprotective, and myeloproliferative effects and therefore might be therapeutically indicated. The histopathological damage caused by methotrexate on liver and kidney was reversed with the treatment of honey and bee venom where the combined treatment had a synergistic effect in restoring the normal histology of both the organs. The findings indicate that honey and bee venom show their equivalent protection against MTX toxicity when combined. These results suggest that they may be useful as adjunct therapeutic agents to reduce the adverse effects of MTX treatment. Molecular mechanisms and clinical validation are yet to be explored in future research.
Keywords: Bee venom, honey, histopathology, kidney function, liver function, Methotrexate toxicity
Graphical abstract:
-
Introduction
Methotrexate (MTX) is a popular chemotherapeutic and immunosuppressive medication used to treat cancer, autoimmune disorders, and inflammation. Despite its therapeutic efficacy, MTX is renowned for its severe toxicity, especially to the liver, kidneys, hematological system, and reproductive functions[1]. The causes of MTX-induced toxicity are oxidative stress, mitochondrial dysfunction, and inflammatory responses leading to hepatocellular damage, nephrotoxicity, and myelosuppression[2]. These severe adverse effects have prompted researchers to come up with alternative or complementary therapeutic strategies to reduce systemic MTX toxicity while retaining its therapeutic benefit. Natural products have attracted increased attention for the therapeutic intervention of drug-induced toxicities. Bee venom and honey have been specifically studied for their immunomodulatory, anti-inflammatory, and antioxidant properties[3]. Natural substances with enzymatic antioxidants, such as honey, polyphenols, and flavonoids, have been known to have hepatoprotective and nephroprotective characteristics by reducing oxidative stress and modulating the inflammatory pathway[4]. Bee venom (melittin, apamin, etc.) the biologically active peptides have been demonstrated to lower levels of inflammatory cytokines and increase the antioxidant defense mechanisms[5]. Honey and bee venom are potential alternative or adjunctive treatments for MTX-induced toxicity due to these properties.
Previous studies have shown that MTX has detrimental effects on organ function. For instance, a study demonstrated that methotrexate (MTX) administration leads to increased levels of liver enzymes such as SGPT, SGOT, and ALP, indicating hepatic damage[6]. As reported by others, elevated levels of creatinine and urea in the serum, along with histopathological changes in kidney tissues, have been observed in MTX-induced nephrotoxicity[7]. The findings underlie the need to develop protective agents against organ toxicity induced by MTX. There have been studies on how natural compounds like bee venom and honey can mitigate MTX toxicity. In addition, the administration of honey has shown to attenuate oxidative stress markers and augment the activities of antioxidant enzymes in methotrexate-treated rats[8]. Bee venom is also thought to have hepatoprotective action by modulating TNF-α and NF-κB pathways that inhibit inflammation and apoptosis of the cell[9]. Honey and bee venom can also restore hematological indices, which adds to their ability to ameliorate MTX-induced myelosuppression[10]. Honey and bee venom have been shown to have individual therapeutic effects, but the combined efficacy of honey and bee venom in the amelioration of MTX-induced toxicity has not been studied much. Moreover, honey and bee venom might have a synergistic effect and enhance their hepatoprotective, and nephroprotective, they may be promising candidates for further investigation. Studies also show that natural products in combination with conventional chemotherapy could afford better protection against systemic toxicity by targeting multiple biochemical pathways[11]. Despite the use of MTX in long-term therapy, methotrexate-induced toxicity remains a major clinical challenge due to severe hepatotoxicity, nephrotoxicity, and hematological suppression. Current protective strategies that have been attempted to avoid MTX-induced organ damage include vitamin folic acid supplementation and antioxidant therapy but are not very effective[12]. It is urgently required to find effective, natural, and complementary therapeutic agents that reduce MTX toxicity while increasing the tolerability of the treatment. Honey's therapeutic potential and bee venom in opposing the toxicity of MTX in albino rats are evaluated from an angle given the established antioxidant and anti-inflammatory properties of honey and bee venom. ‘The protective mechanisms of honey and bee venom could be useful in developing adjunct therapies for patients receiving methotrexate treatment’. Additionally, the biochemical and physiological pathways affected by these natural compounds can help in developing standardized therapeutic approaches to reduce chemotherapy-associated toxicities[13]
This study is designed to achieve the following objectives:
-
To determine the protective effects of honey and bee venom on body weight, liver, and kidney function markers in methotrexate-induced toxicity.
-
To determine the effects of honey and bee venom on MTX-treated albino rats' hematological parameters (WBCs, RBCs, platelets, % hemoglobin (Hb), MCV, MCH, and MCHC).
-
To examine the histopathology of liver and kidney after methotrexate treatment and with therapeutic agents, honey and bee venom
-
To compare the individual and combined efficacy of honey and bee venom in mitigating methotrexate-induced systemic toxicity.
‘By addressing these objectives, this study aims to provide scientific evidence supporting the potential use of honey and bee venom as complementary therapeutic agents in mitigating the adverse effects of methotrexate therapy. Furthermore, the study will contribute to the growing body of research advocating for the integration of natural antioxidants into conventional medicine.
-
Materials and Methods
2.1 Experimental Design
Methotrexate (MTX) induced toxicity in Wistar albino rats and therapeutic effects of honey and bee venom on blood parameters, organ function markers, and histopathology of liver and kidney were studied. The experiments followed ethical rules and were authorized by the Institutional Animal Ethics Committee of Crystal Biologicals, Pune (IAEC Approval No.: CRY/2425/182; Date-16/09/2024) which ensured that animal care standards were met. The study lasted 42 days and evaluated the effects of honey and bee venom on several physiological and biochemical markers in a model of methotrexate-induced toxicity.
2.2 Animals and Housing Conditions
Male Albino rats of Wistar strain, weighing 120-200 g were brought from a licensed animal house at Crystal Biologicals, Pune, and kept in standard laboratory settings. ‘They were housed with 12-hour light, 12-hour dark cycles, and a regulated temperature (22 ± 2°C) with 50-60% relative humidity. They were fed regular pellet feed and provided free access to water during the study. To reduce stress, the animal’s baseline physiological parameters were established by acclimatizing them to Unity for one week before the start of the experimental treatments.
2.3 Experimental Groups and Treatment Regimen
There were 5 experimental groups consisting of 6 rats per group in the study. The first group was the standard control group and was maintained on a standard diet and distilled water 10ml/kg p.o without any treatment. In the second group, the disease control, i.p. injection of Methotrexate (Liquid injection) with Batch no-BYA1002 and purchased from Zydus Lifesciences Limited at a dose of 5 mg/kg weekly once for 42 days was used to induce systemic toxicity, particularly hepatotoxicity, nephrotoxicity, and oxidative stress-related damage. ‘To assess the protective effect of honey against MTX toxicity, the third group received methotrexate (5 mg/kg i.p.) weekly once for 42 days along with honey (1.2 g/kg p.o.) daily which was collected from Scientific Beekeeping Research and Training Centre,Dalsingi Jamner District ,Jalgaon ,Maharashtra.T he fourth group was administered methotrexate (5 mg/kg i.p.) weekly once in 42 days and bee venom (0.5 mg/kg i.p.) daily to see how it affected MTX-induced damage. Bee venom was collected from near about 10 colonies of Apis mellifera in Scientific Beekeeping Research and Training Centre,Dalsingi Jamner District ,Jalgaon ,Maharashtra The fifth group received methotrexate (5 mg/kg i.p.), honey (1.2 g/kg p.o.), and bee venom (0.5 mg/kg i.p.) to investigate the potential synergistic effects of these natural substances in lowering toxicity.
2.4 Induction of Methotrexate-Induced Toxicity
Methotrexate, which is a widely used immunosuppressive and chemotherapeutic agent, is notorious for systemic toxicity caused by its induction of oxidative stress, inflammation, and organ damage. In the MTX-induced toxicity model, rats from various groups were given 5 mg/kg body weight intraperitoneally (i.p.) weekly once for 42 days. This dose was chosen from previous studies that have shown hepatotoxic, nephrotoxic, and reproductive toxic effects of methotrexate[14-16]. Bee venom at 0.5mg/kg daily[2,17] and honey at 1.2g/kg on a daily basis[18,19].
2.5 Body Weight Measurement
Body weight was measured at baseline and on Days 7, 14, 21, 28, 35, and 42 to monitor overall longitudinal health status and systemic toxicity caused by MTX. Significant reductions in body weight in the MTX-treated group compared to the control group indicated toxicity. To determine if honey and bee venom could have therapeutic roles, the effects of honey and bee venom in restoring body weight were analyzed.
2.6 Biochemical Analysis
Blood samples were obtained after 42 days via retroorbital plexus puncture while under moderate anesthesia. The blood samples were centrifuged for 10 minutes at 3,000 rpm to separate the serum, which was then stored at -20 °C until further biochemical examination. Serum glutamate pyruvate transaminase (SGPT), serum glutamate oxaloacetate transaminase (SGOT), alkaline phosphatase (ALP), total protein, at -20 °C, and total bilirubin and albumin were tested to assess liver function using standard biochemical laboratory kits. In addition, serum creatinine, urine, and uric acid were tested to determine renal function. These biochemical indicators revealed the degree of MTX-induced hepatotoxicity and nephrotoxicity, as well as the potential therapeutic effects of honey and bee venom[20].
2.7 Hematological Analysis
An automated hematology analyzer assessed hematological parameters in whole blood samples. The parameters measured included mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), hemoglobin (HGB) levels, hematocrit (HCT), white blood cell (WBC), red blood cell (RBC), and platelet (PLT) counts. ‘Because methotrexate has been shown to produce myelosuppression, hematological analysis was used to investigate the protective effects of honey and bee venom against MTX-induced alterations in blood parameters.
2.8 Absolute Organ Weight and Relative Organ Weight Assessment
The animals were euthanized by CO2 anesthesia after 42 days of treatment, and the liver, and kidneys, were removed carefully, weighed, and fixed for analysis. Organ weight changes were used as an indicator of toxicity, and organ atrophy or hypertrophy was compared between different treatment groups.
2.9 Histopathological Examination
Tissue samples from the liver and kidneys were obtained after euthanasia and preserved in 10% neutral buffered formalin for 48 hours. Tissues were stained with hematoxylin and eosin (H&E) after processing, embedded in paraffin wax, and sectioning done at 4 to 5 μm thickness. ‘After methotrexate, the cellular damage, necrosis, degeneration, and structural changes were analyzed histopathologically under a light microscope, and the protective effects of honey and bee venom were assessed.. ’
2.10 Statistical Analysis
All data were reported as mean ± SD. Number of animals of all experimental groups (n) = 6. Significant differences were observed as *p<0.05, **p<0.01, ***p<0.001, and **** p<0.0001. Values of the Normal Control group were compared against the Disease Control group by Student’s t-test. The values of the Disease Control group were compared against standard and treatment groups by one-way ANOVA followed by Dunnet’s comparison test using GraphPad Prism 9 software.
3. Results and Discussion
3.1 Effect on Body Weight
Methotrexate (MTX) administration was associated with a significant reduction in body weight that resulted in systemic toxicity and metabolic impairment. In later stages, the MTX-treated group lost substantial weight compared to the normal control group. But honey and bee venom supplementation prevented this weight loss, and the combination therapy had the most pronounced effect. Body weight restoration in treated groups supports the hypothesis that honey and bee venom act against MTX-induced metabolic and cachexia-like stress, which might result from antioxidant and anti-inflammatory properties. These results indicate a need for loss prevention of metabolic balance within chemotherapy-induced toxicity models through natural bioactive compounds that play a role in maintaining physiological homeostasis during antifolate treatment.
Table 1 shows the impact of bee venom or honey on body weight change caused by methotrexate (MTX)-induced toxicity in albino rats. Comparing the MTX-treated group to the normal control, the results show a considerable decrease in body weight. Body weight, however, was progressively increased by the administration of honey or venom. While honey alone or bee venom alone was effective in alleviating weight loss incurred by MTX therapy, the combination therapy (honey + bee venom) was the most efficacious and appeared to drive synergistic activity against MTX-related weight loss. These results indicate the protective effect of natural bioactive compounds on maintaining physiological stability.
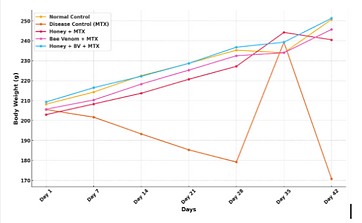
Figure 1: Effect of Honey and Bee Venom on Body Weight Over Time
Figure 1 depicts the impact of bee venom and honey on body weight variations over time in methotrexate (MTX)-induced toxicity. The MTX control group experienced systemic toxicity, as evidenced by a considerable loss in body weight. Significant weight gain was observed following treatment with honey and bee venom, with the combined therapy being the most successful. The data show that honey and bee venom reduce MTX-induced cachexia, most likely due to their antioxidant and anti-inflammatory effects, implying that they may act as preventive agents against chemotherapy-induced weight loss.
3.2 Effect on Biochemical Parameters
Biochemical markers with the potential for hepatotoxicity and nephrotoxicity were significantly affected by methotrexate (MTX) administration. In the MTX-treated group, the liver function enzymes SGPT, SGOT, and ALP were very high and indicated hepatic damage. Total protein and albumin levels dropped concurrently with impaired liver function and increased bilirubin level The kidney function markers increased significantly including creatinine, urea, and uric acid confirming renal toxicity. Honey and bee venom treatment effectively restored biochemical parameters, and the combination therapy (honey + bee venom)offered better protection.. The observed improvements indicate that honey and bee venom-based anti-inflammatory and antioxidant qualities help to buffer the MTX-induced biochemical alterations, making honey and bee venom good protecting agents against drug-induced toxicity.
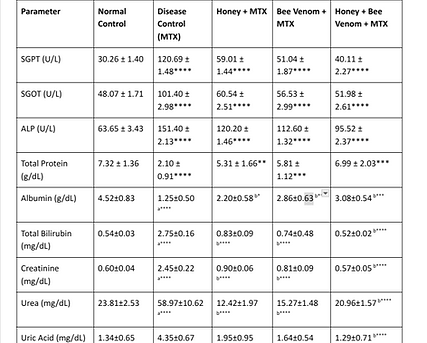
Table 2. Effect of Honey and Bee Venom on Biochemical Parameters
Table 2 further shows biochemical parameters that are under the influence of MTX administration and the protective potential of honey and bee venom in MTX-treated animals. SGPT, SGOT, and ALP,bilirubin in the livers of MTX-treated rats were significantly elevated and indicated hepatotoxicity. Kidney function markers such as creatinine and urea also increased, resulting in nephrotoxicity. Honey treatment, bee venom, and their combination treatments all reduced these biochemical abnormalities dramatically, restoring liver and kidney function. This therapy significantly increased the protective effect over the combination therapy.
Figure 3: Effect of Honey and Bee Venom on Hematological Markers
Figure 3 shows the effect of honey and bee venom treatment on hematological parameters in methotrexate (MTX)-induced toxicity. Significantly, WBC, RBC, hemoglobin, and platelet counts were reduced by MTX but were predominantly stem cell counts, affecting both the myeloid and erythroid cells. The values recovered from treatment with honey and bee venom and were significantly restored by combined therapy, particularly by the combination therapy. Honey and bee venom inhibit MTX-induced hematological suppression with hepatoprotective effects, providing results that strongly indicate the antioxidant and anti-inflammatory mechanisms of these treatments
Changes in organ weight can reflect tissue damage, swelling, or shrinkage. In toxicology studies, organ weight measurements help assess the safety and potential side effects of drugs. They also provide valuable insights into disease progression, such as liver damage or cancer. Overall, organ weight is a useful indicator for understanding the effects of treatments and guiding therapeutic development.
After the administration of MTX, the liver and kidney weights increased in rats of the disease control group. The increase in liver and kidney weights after MTX administration may result from drug-induced toxicity, causing inflammation and compensatory enlargement. Treatment with honey, bee venom, and their combination may counteract these effects through antioxidant, anti-inflammatory, and hepatoprotective properties, promoting organ recovery. The combination group likely showed the best results due to the synergistic effects of both treatments. The effect of honey and bee venom on absolute organ weights in MTX-treated albino mice is depicted in Figure 4 .
Figure 4: Effect of Honey and Bee Venom on Absolute Organ Weights in Methotrexate (MTX)-Treated Albino Rats
Changes in relative organ weight can reflect tissue damage, inflammation, or compensatory enlargement, making it valuable in toxicology studies. These measurements provide insights into drug safety, side effects, and disease progression, such as liver damage.
In this study, MTX administration increased the relative weights of the liver and kidneys, indicating drug-induced toxicity. This likely results from inflammation and compensatory enlargement as these organs work to detoxify the body. The effect of honey and bee venom on relative organ weights in MTX treated rats is illustrated in Figure 5
Figure 5: Effect of Honey and Bee Venom on Relative Organ Weights in Methotrexate (MTX)-Treated Albino Rats
3.4 Histopathology of Liver
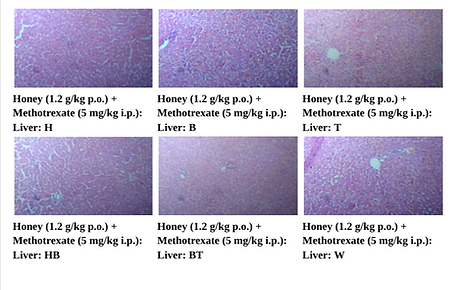
Liver tissues from the methotrexate treated rats were subjected to histological examination, which showed marked hepatocellular degeneration, inflammatory infiltration, necrosis. In contrast, honey and bee venom treatments maintained hepatic architecture and the combination therapy had centrilobular regions near normal, thus providing significant hepatoprotection.
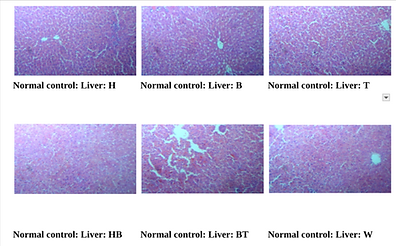
Figure 6. Photographic Representation of Histopathology of Liver Tissue: Normal Control Group Showing normal centrilobular area and hepatic parenchyma. 40X, H & E Stain.
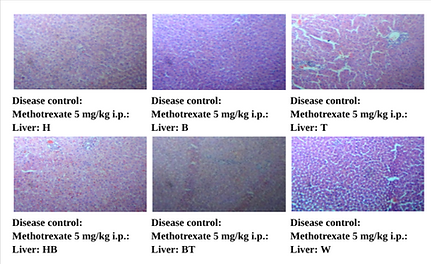
Figure 7. Photographic Representation of Histopathology of Liver Tissue: Disease Control: Methotrexate 5 mg/kg i.p. Group Showing Infiltration of inflammatory cells with hepatocellular degeneration and necrosis, multifocal. 40X, H & E Stain.
Figure 8. Photographic Representation of Histopathology of Liver Tissue: Honey (1.2 g/kg p.o.) + Methotrexate (5 mg/kg i.p.) Group showing normal centrilobular area and hepatic parenchyma. 40X, H & E Stain.
Figure 9. Photographic Representation of Histopathology of Liver Tissue: Bee Venom (0.5 mg/kg i.p.) + Methotrexate (5 mg/kg i.p.) Group Showing normal centrilobular area and hepatic parenchyma. 40X, H & E Stain.
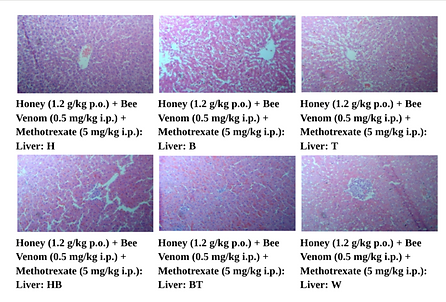
Figure 10. Photographic Representation of Histopathology of Liver Tissue: Honey (1.2 g/kg p.o.) + Bee Venom (0.5 mg/kg i.p.) + Methotrexate (5 mg/kg i.p.) Group Showing normal centrilobular area and hepatic parenchyma.40X,H×E stain.
(H, B, T, HB, BT, W-Animal identification markings: H-Head, B-Back, T-Tail, HB-Head Back, BT-Back Tail, W-White)
3.5 Histopathology of Kidney
MTX treatment of animals resulted in pronounced tubular degeneration, interstitial inflammation and cortical-medullary damage in kidney sections. These lesions were ameliorated by treatment with honey, bee venom, or their combination, and the combination group had well preserved renal tubules and reduced inflammatory infiltration confirming nephroprotective efficacy.
Figure 11. Photographic Representation of Histopathology of Kidney Tissue: Normal Control Group Showing normal renal tubules in cortex and medulla. 40X, H & E Stain.
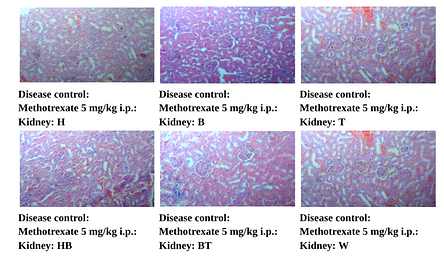
Figure 12. Photographic Representation of Histopathology of Kidney Tissue: Disease Control: Methotrexate 5 mg/kg i.p. Group Showing Infiltration of inflammatory cells in cortex and medulla, multifocal. 40X, H & E Stain.
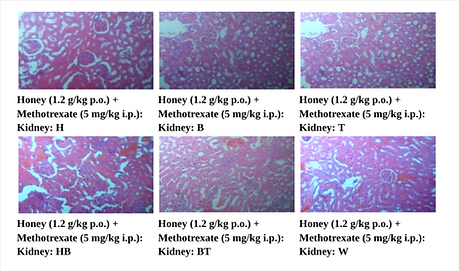
Figure 13. Photographic Representation of Histopathology of Kidney Tissue: Honey (1.2 g/kg p.o.) + Methotrexate (5 mg/kg i.p.) Group showing normal renal tubules in the cortex and medulla. 40X, H & E Stain.
Figure 14. Photographic Representation of Histopathology of Kidney Tissue: Bee Venom (0.5 mg/kg i.p.) + Methotrexate (5 mg/kg i.p.) Group showing normal renal tubules in the cortex and medulla. 40X, H & E Stain.
Figure 15. Photographic Representation of Histopathology of Kidney Tissue: Honey (1.2 g/kg p.o.) + Bee Venom (0.5 mg/kg i.p.) + Methotrexate (5 mg/kg i.p.) Group showing normal renal tubules in the cortex and medulla. 40X, H & E Stain
(H, B, T, HB, BT, W-Animal identification markings: H-Head, B-Back, T-Tail, HB-Head Back, BT-Back Tail, W-White)
The results of this investigation revealed that honey and bee venom were very protective against methotrexate (MTX)-induced toxicity in albino rats. Body weight reductions to the extent of 90%, enhanced liver and kidney function markers, and hematological suppression was observed with the administration of MTX. Although treatment of honey and bee venom improved these physiological and biochemical indices significantly, honey and bee venom combination therapy was the best and restored the biochemical, and hematological close to normal control values. These results indicate the therapeutic application of natural bioactive compounds to reduce drug toxicity. Honey and bee venom thus appear to protect body weight maintenance in a cachexia-like state induced by methotrexate as possible mechanisms of action seem to be by their anti-inflammatory and metabolic regulatory properties. It also shows the results of the improvement in the liver function markers, such as the SGPT and SGOT, which are hepatoprotective effects that may be due to the antioxidant components in honey and bee venom, reducing oxidative stress and hepatic injury.
The study's findings are consistent with previous research revealing the antioxidant and anti-inflammatory properties of honey and bee venom. Bee venom was found to reduce TNF-α and NF-κB activation, thereby protecting the liver against MTX-induced inflammation and damage6. Mistletoe extract has been shown to reduce MTX-associated nephrotoxicity through its antioxidant mechanism7. Supplemented by previous findings8, the observed restoration of hematological indices supports the cytoprotective effects of natural agents in mitigating MTX-induced myelosuppression. The findings of this study are compared with other reports, and it is evident that natural compounds such as honey and bee venom are important in reducing systemic toxicity and oxidative damage21. Honey's impact on the metabolism of carbohydrates and fat has also been mentioned by several studies, Honey has potent inflammatory, immunomodulatory, and antimicrobial properties. All of these properties might contribute to the hepatoprotective and nephroprotective effects19. Like bee venom, bee venom is known to activate endogenous antioxidant pathways, decreasing lipid peroxidation and protecting cell integrity. These results are consistent with other evidence that bioactive natural products can mitigate the adverse effects of chemotherapeutic agents such as methotrexate.
Methotrexate (MTX) administration leads to enlarged liver and kidney weights due to inflammation, cellular infiltration, and oxidative stress[22]. Organ hypertrophy appears as a protective mechanism when tissue damage occurs. The results match these findings because MTX-treated rats showed increased weights for both absolute and relative organs[23]. Recent evidence shows that honey and bee venom treatments together produced the most significant reversal effect on these changes[24] because both agents possess potent antioxidant and anti-inflammatory properties. The histopathological analysis confirmed these results by showing that MTX induced hepatocellular necrosis, sinusoidal dilation, and tubular degeneration in the kidneys[25]. The analysis revealed that honey and bee venom protected liver lobular structure and renal tubules from damage, thus demonstrating histoprotective benefits26. The experimental data support the utilization of natural bioactive substances as a solution for preventing organ damage caused by MTX treatment.
These findings have important implications in both clinical and pharmacological applications. As increasing demands of drug-induced organ toxicity emerge, the use of honey and bee venom may potentially act as complementary therapeutic agents in reducing the toxicity associated with the use of chemotherapeutic and immunosuppressive drugs such as methotrexate. These results also support the formulation of novel formulations with honey and bee venom to have hepatoprotective and nephroprotective benefits for their reproductive health. This also adds to an existing literature that is increasingly recommending natural antioxidants be included in conventional medical treatments to increase both drug safety and efficacy. Seeing if honey and bee venom-producing efficacy against methotrexate toxicity would, from a pharmacological perspective, be worthy of consideration as an adjunct therapy. Such an effort in designing novel herbal formulations to maximize the treatment expenditures by blocking organ damage and optimizing the tolerability of chemotherapy in patients needing long-term methotrexate therapy would be helpful. In addition, these results open up new avenues for studying natural compounds to prevent drug-induced oxidative damage and tissue injury to ultimately enhance treatment outcomes and patient quality of life.
This study has some limitations despite promising results. The sample size was small enough, although it might not influence the generalizability of the findings. Specifically, biochemical and histopathological analysis were undertaken, but molecular investigations on the signaling pathways modulated by honey and bee venom were not performed. There is still further research using gene expression and protein analysis that would help elucidate the exact mechanism of action. Furthermore, the study was performed in an animal model, and these findings are encouraging, but clinical trials in human populations are required to validate the therapeutic efficacy of honey and bee venom in reducing MTX toxicity. A limitation of this study is that there are no long-term toxicity assessments for honey and bee venom. However, long-term administration has yet to be investigated on the other side, and safety still needs to be determined. Also, compositional variations of honey and bee venom in the context of environment and region may affect their bioactivity, and therefore, they require standardization and control of quality before their use as a therapeutic.
The scope of this research should be expanded to future studies, which could include larger sample sizes and biochemical and molecular studies to further examine the mechanistic pathways by which the protective actions of honey and bee venom occur. Also, investigating the effect of these natural compounds combined would yield new insights into synergistic effects. Additionally, clinical trials should be performed to determine the efficacy and safety of honey and bee venom in patients receiving methotrexate therapy. To accelerate their adoption, it might be possible to standardize dosage formulations and explore the long-term effects. Future directions of this study will increase the translational potential of this study and, therefore, expand the use of natural therapeutics in modern medicine. Further research should also investigate the immunomodulatory effects of honey and bee venom on methotrexate-induced toxicity. Since such natural agents have been shown to prevent or resolve kidney and other drug-induced organ damage, and since inflammatory pathways play a critical role in mediating this type of organ damage, it may be that the immunological changes induced by these natural agents provide valuable information on mechanisms for preventing or resolving drug-induced organ damage. The interactions of these organisms with conventional pharmaceuticals may also be searched for drug synergistic effects, thus broadening their use in clinical settings further.
4. CONCLUSION
The findings of this study point to the possible therapeutic application of honey and bee venom in decreasing methotrexate (MTX)-induced toxicity in albino rats’. MTX administration was linked to severe hepatotoxicity, nephrotoxicity, and hematological suppression. Although treatment with honey and bee venom did not lead to significant improvements in biochemical and hematological treatment with honey and bee venom in combination did, and this was shown to protect these parameters as well as preserve the normal histopathological architecture of the liver and kidney tissues, as observed under microscopic analysis. The importance of natural bioactive compounds to reduce drug toxicity and improve overall physiological functions is underscored in these findings. ‘Honey has antioxidant and anti-inflammatory effects, and bee venom is the reason for their hepatoprotective and nephroprotective effects’. Also, their capacity to restore hematological indices further supports their cytoprotective role, and they are promising candidates to ameliorate MTX-induced myelosuppression. Further research indicates that natural compounds are effective in combating chemotherapeutic toxicity, and this study is aligned with the previous research. Studies on the molecular mechanism, optimal dosage, and safety are needed further. This translational research will need to be expanded to human clinical trials to validate the translational potential of honey and bee venom as adjunct therapies for MTX-induced toxicity. Thus, honey and bee venom possess promising protective effects against MTX-induced toxicity and are potential complementary therapeutics for individuals on methotrexate treatment. Such integration in clinical practice may improve treatment efficacy without adverse effects. Molecular studies and clinical validation to establish standardized therapeutic protocols that are safe and effective for their use will be the focus of future research.
References
1 Attia, S. H., Elshazly, S. M., Abdelaal, M. M., & Soliman, E. (2022). Reno-protective effect of mangiferin against methotrexate-induced kidney damage in male rats: PPARγ-mediated antioxidant activity. Saudi pharmaceutical journal : SPJ : the official publication of the Saudi Pharmaceutical Society, 30(9), 1252–1261. https://doi.org/10.1016/j.jsps.2022.06.026
2 Meligi, N. M., Ismail, S. A., & Tawfik, N. S. (2020). Protective effects of honey and bee venom against lipopolysaccharide and carbon tetrachloride-induced hepatoxicity and lipid peroxidation in rats. Toxicology research, 9(5), 693–705. https://doi.org/10.1093/toxres/tfaa077
3 Bogdanov, S. (2017). Biological and therapeutic properties of bee venom. In The Bee Venom Book (pp. 1-24). Bee Product Science.
4 Pillai, S. S., & Waykar, B. B. (2024). Ameliorative role of honey and bee venom of Apis mellifera against methotrexate-induced alterations on biochemical parameters of liver and kidney in male Wistar albino rats. Journal of Applied Bioanalysis, 10(3), 210-220
5 El-Tedawy DM, Abd-Alhaseeb, M. M., Helmy, M. W., & Ghoneim, A. I. (2020). Systemic bee venom exerts anti-arthritic and anti-inflammatory properties in a rat model of arthritis. Biomedical Reports, 13(2), 15. https://doi.org/10.3892/br.2020.1327
6 Darwish, S. F., El-Bakly, W. M., Arafa, H. M., & El-Demerdash, E. (2013). Targeting TNF-α and NF-κB activation by bee venom: role in suppressing adjuvant-induced arthritis and methotrexate hepatotoxicity in rats. PLoS ONE, 8(11), e79284. https://doi.org/10.1371/journal.pone.0079284
7 Çetin, E. S., Tetiker, H., Çelik, Ö. İ., Yılmaz, N., & Ciğerci, İ. H. (2017). Methotrexate-induced nephrotoxicity in rats: Protective effect of mistletoe (Viscum album L.) extract. Complementary Medicine Research, 24(6), 364-370. https://doi.org/10.1159/000468984
8 Rizk, F. H., Saadany, A. A. E., Dawood, L., Elkaliny, H. H., Sarhan, N. I., Badawi, R., & Abd-Elsalam, S. (2018). Metformin ameliorated methotrexate-induced hepatorenal toxicity in rats in addition to its antitumor activity: two birds with one stone. Journal of inflammation research, 11, 421–429. https://doi.org/10.2147/JIR.S178767
9 Stela, M., Cichon, N., Spławska, A., Szyposzynska, M., & Bijak, M. (2024). Therapeutic Potential and Mechanisms of Bee Venom Therapy: A Comprehensive Review of Apitoxin Applications and Safety Enhancement Strategies. Pharmaceuticals (Basel, Switzerland), 17(9), 1211. https://doi.org/10.3390/ph17091211
10 Widemann, B. C., Balis, F. M., Kempf-Bielack, B., Bielack, S., Pratt, C. B., Ferrari, S., Bacci, G., Craft, A. W., & Adamson, P. C. (2004). High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer, 100(10), 2222–2232. https://doi.org/10.1002/cncr.20255
11 Schmidt, S., Messner, C. J., Gaiser, C., Hämmerli, C., & Suter-Dick, L. (2022). Methotrexate-Induced Liver Injury Is Associated with Oxidative Stress, Impaired Mitochondrial Respiration, and Endoplasmic Reticulum Stress In Vitro. International journal of molecular sciences, 23(23), 15116. https://doi.org/10.3390/ijms232315116
12 Namjou, A., Yazdani, N., Rafieian-Kopaei, M., & Eskandari, Y. (2022). Effects benefits and hazards of honey bee venom on wound healing and serum biochemical changes in alloxan-induced diabetic rats. Comparative Clinical Pathology, 31(4), 659-668. http://dx.doi.org/10.1007/s00580-022-03363-z
13 Bava, R., Castagna, F., Musella, V., Lupia, C., Palma, E., & Britti, D. (2023). Therapeutic Use of Bee Venom and Potential Applications in Veterinary Medicine. Veterinary sciences, 10(2), 119. https://doi.org/10.3390/vetsci10020119
14 Cianciosi, D., Forbes-Hernández, T. Y., Afrin, S., Gasparrini, M., Reboredo-Rodriguez, P., Manna, P. P., Zhang, J., Bravo Lamas, L., Martínez Flórez, S., Agudo Toyos, P., Quiles, J. L., Giampieri, F., & Battino, M. (2018). Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules (Basel, Switzerland), 23(9), 2322. https://doi.org/10.3390/molecules23092322
16 Ghoneum, M., & El-Gerbed, M. S. A. (2021). Human placental extract ameliorates methotrexate-induced hepatotoxicity in rats via regulating antioxidative and anti-inflammatory responses. Cancer chemotherapy and pharmacology, 88(6), 961–971. https://doi.org/10.1007/s00280-021-04349-4
17 Ahmed, Z. S. O., Hussein, S., Ghandour, R. A., Azouz, A. A., & El-Sakhawy, M. A. (2021). Evaluation of the effect of methotrexate on the hippocampus, cerebellum, liver, and kidneys of adult male albino rat: Histopathological, immunohistochemical and biochemical studies. Acta Histochemica, 123(2), 151682.
18 Zahran, F., Mohamed, A., & Zein, N. (2021). Bee venom attenuates degenerative effects of diabetes associated with hyperlipidemia in rats. Biochemistry Letters, 17(1), 77-107.
19 Alturkistani, H. A., Abuzinadah, O. A., Kelany, A. M., Aziz, G. S., & Alrafiah, A. R. (2019). The combined effect of honey and olive oil against methotrexate mediated hepatotoxicity in rats: A biochemical, histological and immunohistological study.
20 Sadek, K. M., Shib, N. A., Taher, E. S., Rashed, F., Shukry, M., Atia, G. A., Taymour, N., El-Nablaway, M., Ibrahim, A. M., Ramadan, M. M., Abdelkader, A., Abdo, M., Imbrea, I., Pet, E., Ali, L. S., & Abdeen, A. (2024). Harnessing the power of bee venom for therapeutic and regenerative medical applications: an updated review. Frontiers in pharmacology, 15, 1412245. https://doi.org/10.3389/fphar.2024.1412245
21 Kumar, J. B., Goud, B. M., & Kumar, A. (2021). Liver function tests: biochemical overview for clinical correlation. hormones, 25, 26. http://dx.doi.org/10.5005/jp-journals-10054-0171
22 Dennis, J. M., & Witting, P. K. (2017). Protective Role for Antioxidants in Acute Kidney Disease. Nutrients, 9(7), 718. https://doi.org/10.3390/nu9070718
23 Moodi, H., Hosseini, M., Abedini, M. R., Hassanzadeh-Taheri, M., & Hassanzadeh-Taheri, M. (2020). Ethanolic extract of Iris songarica rhizome attenuates methotrexate-induced liver and kidney damages in rats. Avicenna journal of phytomedicine, 10(4), 372–383.
24 Yang, L., He, X., Xue, Y., Zhi, D., Meng, Q., Zhao, W., Gong, X., Yue, D., Dong, K., & Tian, Y. (2024). Amelioration of melittin on adjuvant-induced rheumatoid arthritis: Integrated transcriptome and metabolome. International journal of biological macromolecules, 270(Pt 1), 132293. https://doi.org/10.1016/j.ijbiomac.2024.132293
25 Chung, E. S., Lee, G., Lee, C., Ye, M., Chung, H. S., Kim, H., Bae, S. J., Hwang, D. S., & Bae, H. (2015). Bee Venom Phospholipase A2, a Novel Foxp3+ Regulatory T Cell Inducer, Protects Dopaminergic Neurons by Modulating Neuroinflammatory Responses in a Mouse Model of Parkinson's Disease. Journal of immunology (Baltimore, Md.: 1950), 195(10), 4853–4860. https://doi.org/10.4049/jimmunol.1500386
26 Howard, S. C., McCormick, J., Pui, C. H., Buddington, R. K., & Harvey, R. D. (2016). Preventing and Managing Toxicities of High-Dose Methotrexate. The oncologist, 21(12), 1471–1482. https://doi.org/10.1634/theoncologist.2015-0164
27 Helal, M. G., & Said, E. (2020). Tranilast attenuates methotrexate-induced renal and hepatic toxicities: Role of apoptosis-induced tissue proliferation. Journal of biochemical and molecular toxicology, 34(5), e22466. https://doi.org/10.1002/jbt.22466






.png)